


Phytochemical profile and biological properties of pulp and peel extracts of Docynia indica (Wall.) Decne – An endemic wild fruit of North East India
Sangeetha S T1, Sheila John1*, Sarah Jane Monica2, Sivaraj C3, Arumugam P3
1 Department of Home Science, Women’s Christian College (Autonomous) Chennai, Tamil Nadu, India
2 Department of Nutrition, Food Service Management and Dietetics, Ethiraj College for Women (Autonomous) Chennai, Tamil Nadu, India
3 Armats Biotek Training and Research Institute, Chennai, Tamil Nadu, India.
ABSTRACT
Background: Different parts of wild fruit varieties grown in North East India have a diverse range of functional components that impart several health benefits. With a focus to stimulate maximum utilization of fruit crops, the aim of the research work was to estimate the composition of phytochemicals, screen functional compounds, and determine the biological properties of pulp and peel extracts of Docynia indica. Materials and methods: Phytochemicals were quantified using standard methods. Antioxidant activity was analyzed using DPPH●, Fe3+ reducing and phosphomolybdenum reduction method. Antibacterial activity was determined using a well diffusion assay against 6 bacterial strains. Active compounds were identified using GC-MS. Results: Peel extract exhibited remarkable antioxidant activity. Polyphenols function as oxidizing and reducing agents. Peel extract had a higher concentration of phenol and flavonoid. TPC was 313.35±4.03μg/mg GAE and TFC was 121.55±3.66μg/mg QE. Compounds such as coumarin, phenols, fatty acids, pyrazole, flavone and flavone derivates were identified in peel extract. On comparing the results of antibacterial activity, the pulp extract possessed a stronger ability to inhibit the growth of pathogenic bacteria. Maximum inhibition was observed for Staphylococcus aureus with a ZOI of 29 mm at 625µg/mL. Conclusion: The study supports the likelihood of using wild edible fruits as potential functional foods. Furthermore, the peels of wild edible fruits can be efficiently utilized as a natural antioxidant and antimicrobial agents.
Keywords: North east India, wild fruit species, Docynia indica, antibacterial, antioxidant activity
Wild fruits refer to fruits that are neither domesticated nor cultivated, instead grow in their natural wild habitat (Chakravarty et al. 2016). Wild fruits are important sources of essential nutrients such as dietary fibre, non-nutritive constituents, vitamins and minerals for indigenous population (Sundriyal et al. 2004; Bvenura and Sivakumar 2017). Current research investigations indicate the use of different wild fruits in treating cardiovascular diseases, type 2 diabetes mellitus, urinary tract infections, digestive and inflammatory disorders (Li et al. 2016) The North East region of India particularly Manipur is bestowed with extensive diversity of endemic wild fruits and vegetables. Manipur is located at 23.80º - 25.68º N latitude and 93.03º - 94.78 º E longitude. Apart from major and trivial fruit crops that grow in Manipur, other fruit crops such as Calamus tenius, Celtis timorensis, Euphorbia longan, Dillinia indica, Syzygium cumini, Flacourtia jangomas and Rubus moluccanus grow in the forest areas as wild underutilized minor fruits. These fruits are used as medicine or food by the tribal communities (Singh et al. 2016). However, most of them still remain unfamiliar and under exploited despite their nutritional and nutraceutical properties.
Green, pink and red apples produced in northern regions of India are commonly consumed by individuals of all age groups (Rymbai et al. 2016). Wild apple (Docynia indica) is an endemic wild fruit that grows in the hilly areas of north east India. The fruit belongs to Rosaceae family and differs from the normal cultivated apples as the size is about one third the size of its counterparts. They are ellipsoid or sub-globose in shape, greenish or yellowish in colour and taste sweet or slightly bitter. Wild apples grow in Manipur and Meghalaya. They are consumed by the tribal communities either in fresh or unripe form (Boyer and Liu 2004). Traditionally, wild apples were used for treating different infectious diseases (Van 1997). Phenols, flavonoids and alkaloids extracted from wild apple contribute to several medicinal properties. Wild apples cure heart burns, act as an appetite stimulant and exhibit antidiabetic, anti-hypertensive, anti-microbial and anti-obesity effect (Loan et al. 2011; Punjabi et al. 2017).
Apples are rich in phytonutrients. In India, the delicious varieties of apples are produced in Jammu Kashmir, Uttarkhand and Himachal Pradesh (Dhyani et al. 2018). According to literature, more nutrients are present in pericarp than flesh. Concentrations of these nutrients depend upon variety, agro- environmental and biological factors (Kalinowska et al. 2013; Massini et al. 2013). Apples are processed into different products such as juice, sauce, jellies, cider, canned and dried apples. These products are available all throughout the year. In large scale production of apple based processed products, apple peels generated as by-products are not utilized properly. Since apple peels possess different active compounds, these by-products obtained from fruit processing units can be systematically screened for beneficial properties and their potential applications.
Numerous research findings are available to support the medicinal properties of apples cultivated in the northern regions of India. However, there is paucity of information on the therapeutic properties of apples that grow in North East regions of India. The study was conducted to screen bioactive compounds, antioxidant and antimicrobial activity of pulp and peel extracts of wild apple. The results of the study will provide knowledge on phytochemical and health attributes of endemic wild edible fruits grown across different altitudinal ranges. In addition, results on functional properties of fruit peel extract will enable researchers to carry out innovative studies on utilization of biological waste substrates such as peels and seeds in different industries.
Preparation of extract:
Wild apples were bought from Manipur. The fruits were washed with tap water and finally rinsed with deionised water. Peels were separated from the fruit using a sharp sterile knife. Extracts were prepared by soaking 50 g of fresh fruit and fresh peels separately in 100 mL of ethanol. The infusions were left in an orbital shaker for a period of 72 hours. The extracts were filtered using Whatmann filter paper 1. Solvents were removed under reduced pressure. Later, the extracts were dissolved in known volume of solvents. Ethanolic extracts were used for further analysis.
Qualitative analysis of phytochemicals:
Alkaloids, glycosides, flavonoids, phenols, saponins, steroids, tannins terpenoids, and quinones were screened using standard methods (Raaman 2006).
Estimation of TPC:
Folin-Ciocalteau reagent method was used for estimation of TPC (Singleton et al. 1999). Aliquots of the extracts (0.1mL) were mixed with 1000 μL of Folin Ciocalteu reagent. Later, 1 mL of 20% Na2CO3 was added and the solution was incubated for 30 minutes. The absorbance value was measured at 760 nm. TPC are expressed as gallic acid equivalent (μg/mg).
Estimation of TFC:
Aluminium chloride method was used for estimation of TFC (Chang et al. 2002). Aliquots of the extracts (500 μL) were mixed with 0.5 mL of 5% NaNO3 and incubated for 5 minutes. Later, 0.3 mL of 10% aluminium chloride solution was added. After 5 minutes, 1 mL of 1M NaOH was added and the volume was diluted to 5 mL using distilled water. The solution was allowed to stand for 15 minutes. Absorbance value was measured at 510 nm. TFC are expressed as quercetin equivalent (μg/mg).
Antioxidant activity:
Absorbance value of antioxidant assays was measured using UV visible spectrophotometer (Elico 104 - Uvsar)
DPPH● radical scavenging assay:
DPPH● activity was determined using the method given by (Blois 1958). Briefly, 1 mL of 0.1 mM prepared DPPH● solution was added to various concentrations of the extracts. The solutions were left in dark for 30 minutes. Absorbance value was measured at 517 nm. Results are expressed as percentage inhibition of DPPH● free radical.
Ferric (Fe3+) reducing power activity:
Fe3+ reducing power was determined according to the method given by Yen and Chen (1995). Various concentrations of the extracts were mixed with 1 mL of freshly prepared phosphate buffer (0.2 M, pH 6.6) and 1mL of 1% K3[Fe(CN)6]. The reaction mixture was incubated for 20 minutes at 50oC. After cooling the mixture, 500 μL of 10% TCA and 500μL of 0.1% freshly prepared FeCl3 was added. Results are expressed as absorbance value measured at 700 nm.
Phosphomolybdenum reduction assay:
Total antioxidant activity of the extracts was evaluated using phosphomolybdenum method (Prieto et al. 1999). Different concentrations of the extracts were mixed with 1000 μL of the reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. The tubes were incubated at 95°C for about 90 minutes and cooled later. Results are expressed as absorbance value measured at 695 nm. Standard used for all antioxidant assays was ascorbic acid.
Antibacterial activity:
Bacterial pathogens such as Escherichia coli (MTCC 443), Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 96), Shigella flexneri (MTCC 1457), Proteus vulgaris (MTCC 426) and Micrococcus luteus (MTCC 1538) were used to determine the antibacterial activity. The antibacterial activity was determined using well diffusion assay (Langfield et al. 2004). Freshly prepared nutrient agar was poured into the petri plates and was allowed to solidify. The bacterial culture was streaked on the petri plates using a cotton swab aseptically. Wells were created in the petri plates using a sterile steel borer. The extracts were loaded into the wells. The inoculated plates were incubated for about 24 hours. Results are expressed as zone of inhibition in millimetres. Standard used for antibacterial activity was tetracycline.
GC-MS:
The extracts were injected into a HP-5 column (30 m X 0.25 mm with 0.25 μm film thickness). Helium was used as the carrier gas for gas chromatography at a constant rate of 1 mL/minute. The injector was operated at 200°C and the temperature was maintained within 50-250°C at 10°C/min injection mode. Following MS conditions were utilized: ionization voltage of 70 eV, ion source temperature of 250°C, interface temperature of 250°C and mass range of 50-600 mass units (Sivaraj et al. 2019). NIST database was used for interpretation of compounds.
Phytochemical profile:
Secondary plant metabolites are distributed widely in different varieties of fruits and vegetables. In this study, qualitative analysis of phytochemicals report the presence of alkaloids, phenols, flavonoids, glycosides, saponins, tannins and quinones in both the extracts. Terpenoids were detected only in peel extract and cardiac glycosides were found only in pulp extract. TPC and TFC of the extracts were estimated using the regression equation obtained from the calibration curve of standards used for quantification of TPC and TFC. TPC and TFC of pulp extract were found to be 106.20 ± 1.13µg/mg GAE and 56.89 ± 3.66µg/mg QE respectively. TPC of peel extract was nearly three times greater (313.35 ± 4.03 µg/mg GAE) than the pulp extract. Similarly, TFC of peel extract was twice the amount (121.55 ± 3.66 µg/mg GAE) when compared to pulp extract.
Antioxidant activity:
Antioxidant activity of pulp and peel extracts of Docynia indica was determined using in vitro antioxidant assays (Table 1 and Table 2). Although, DPPH● radical scavenging potential was observed in both the extracts, the peel extract showed stronger activity as the IC50 value was 23.76 ± 3.38 µg/mL. Maximum inhibition was 91.65±0.06% at 60µg/mL. The IC50 value of pulp extract was 162.70 ±11.22µg/mL. A similar trend of results was noted for reducing power. Reducing power of the extracts was determined using Fe3+ reduction and phosphomolybdenum reduction methods. The reducing power of peel extract was high when compared to the pulp extract.
Wild fruits refer to fruits that are neither domesticated nor cultivated, instead grow in their natural wild habitat (Chakravarty et al. 2016). Wild fruits are important sources of essential nutrients such as dietary fibre, non-nutritive constituents, vitamins and minerals for indigenous population (Sundriyal et al. 2004; Bvenura and Sivakumar 2017). Current research investigations indicate the use of different wild fruits in treating cardiovascular diseases, type 2 diabetes mellitus, urinary tract infections, digestive and inflammatory disorders (Li et al. 2016) The North East region of India particularly Manipur is bestowed with extensive diversity of endemic wild fruits and vegetables. Manipur is located at 23.80º - 25.68º N latitude and 93.03º - 94.78 º E longitude. Apart from major and trivial fruit crops that grow in Manipur, other fruit crops such as Calamus tenius, Celtis timorensis, Euphorbia longan, Dillinia indica, Syzygium cumini, Flacourtia jangomas and Rubus moluccanus grow in the forest areas as wild underutilized minor fruits. These fruits are used as medicine or food by the tribal communities (Singh et al. 2016). However, most of them still remain unfamiliar and under exploited despite their nutritional and nutraceutical properties.
Green, pink and red apples produced in northern regions of India are commonly consumed by individuals of all age groups (Rymbai et al. 2016). Wild apple (Docynia indica) is an endemic wild fruit that grows in the hilly areas of north east India. The fruit belongs to Rosaceae family and differs from the normal cultivated apples as the size is about one third the size of its counterparts. They are ellipsoid or sub-globose in shape, greenish or yellowish in colour and taste sweet or slightly bitter. Wild apples grow in Manipur and Meghalaya. They are consumed by the tribal communities either in fresh or unripe form (Boyer and Liu 2004). Traditionally, wild apples were used for treating different infectious diseases (Van 1997). Phenols, flavonoids and alkaloids extracted from wild apple contribute to several medicinal properties. Wild apples cure heart burns, act as an appetite stimulant and exhibit antidiabetic, anti-hypertensive, anti-microbial and anti-obesity effect (Loan et al. 2011; Punjabi et al. 2017).
Apples are rich in phytonutrients. In India, the delicious varieties of apples are produced in Jammu Kashmir, Uttarkhand and Himachal Pradesh (Dhyani et al. 2018). According to literature, more nutrients are present in pericarp than flesh. Concentrations of these nutrients depend upon variety, agro- environmental and biological factors (Kalinowska et al. 2013; Massini et al. 2013). Apples are processed into different products such as juice, sauce, jellies, cider, canned and dried apples. These products are available all throughout the year. In large scale production of apple based processed products, apple peels generated as by-products are not utilized properly. Since apple peels possess different active compounds, these by-products obtained from fruit processing units can be systematically screened for beneficial properties and their potential applications.
Numerous research findings are available to support the medicinal properties of apples cultivated in the northern regions of India. However, there is paucity of information on the therapeutic properties of apples that grow in North East regions of India. The study was conducted to screen bioactive compounds, antioxidant and antimicrobial activity of pulp and peel extracts of wild apple. The results of the study will provide knowledge on phytochemical and health attributes of endemic wild edible fruits grown across different altitudinal ranges. In addition, results on functional properties of fruit peel extract will enable researchers to carry out innovative studies on utilization of biological waste substrates such as peels and seeds in different industries.
Preparation of extract:
Wild apples were bought from Manipur. The fruits were washed with tap water and finally rinsed with deionised water. Peels were separated from the fruit using a sharp sterile knife. Extracts were prepared by soaking 50 g of fresh fruit and fresh peels separately in 100 mL of ethanol. The infusions were left in an orbital shaker for a period of 72 hours. The extracts were filtered using Whatmann filter paper 1. Solvents were removed under reduced pressure. Later, the extracts were dissolved in known volume of solvents. Ethanolic extracts were used for further analysis.
Qualitative analysis of phytochemicals:
Alkaloids, glycosides, flavonoids, phenols, saponins, steroids, tannins terpenoids, and quinones were screened using standard methods (Raaman 2006).
Estimation of TPC:
Folin-Ciocalteau reagent method was used for estimation of TPC (Singleton et al. 1999). Aliquots of the extracts (0.1mL) were mixed with 1000 μL of Folin Ciocalteu reagent. Later, 1 mL of 20% Na2CO3 was added and the solution was incubated for 30 minutes. The absorbance value was measured at 760 nm. TPC are expressed as gallic acid equivalent (μg/mg).
Estimation of TFC:
Aluminium chloride method was used for estimation of TFC (Chang et al. 2002). Aliquots of the extracts (500 μL) were mixed with 0.5 mL of 5% NaNO3 and incubated for 5 minutes. Later, 0.3 mL of 10% aluminium chloride solution was added. After 5 minutes, 1 mL of 1M NaOH was added and the volume was diluted to 5 mL using distilled water. The solution was allowed to stand for 15 minutes. Absorbance value was measured at 510 nm. TFC are expressed as quercetin equivalent (μg/mg).
Antioxidant activity:
Absorbance value of antioxidant assays was measured using UV visible spectrophotometer (Elico 104 - Uvsar)
DPPH● radical scavenging assay:
DPPH● activity was determined using the method given by (Blois 1958). Briefly, 1 mL of 0.1 mM prepared DPPH● solution was added to various concentrations of the extracts. The solutions were left in dark for 30 minutes. Absorbance value was measured at 517 nm. Results are expressed as percentage inhibition of DPPH● free radical.
Ferric (Fe3+) reducing power activity:
Fe3+ reducing power was determined according to the method given by Yen and Chen (1995). Various concentrations of the extracts were mixed with 1 mL of freshly prepared phosphate buffer (0.2 M, pH 6.6) and 1mL of 1% K3[Fe(CN)6]. The reaction mixture was incubated for 20 minutes at 50oC. After cooling the mixture, 500 μL of 10% TCA and 500μL of 0.1% freshly prepared FeCl3 was added. Results are expressed as absorbance value measured at 700 nm.
Phosphomolybdenum reduction assay:
Total antioxidant activity of the extracts was evaluated using phosphomolybdenum method (Prieto et al. 1999). Different concentrations of the extracts were mixed with 1000 μL of the reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. The tubes were incubated at 95°C for about measured at 695 nm. Standard used for all antioxidant assays was ascorbic acid.
Antibacterial activity:
Bacterial pathogens such as Escherichia coli (MTCC 443), Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 96), Shigella flexneri (MTCC 1457), Proteus vulgaris (MTCC 426) and Micrococcus luteus (MTCC 1538) were used to determine the antibacterial activity. The antibacterial activity was determined using well diffusion assay (Langfield et al. 2004). Freshly prepared nutrient agar was poured into the petri plates and was allowed to solidify. The bacterial culture was streaked on the petri plates using a cotton swab aseptically. Wells were created in the petri plates using a sterile steel borer. The extracts were loaded into the wells. The inoculated plates were incubated for about 24 hours. Results are expressed as zone of inhibition in millimetres. Standard used for antibacterial activity was tetracycline.
GC-MS:
The extracts were injected into a HP-5 column (30 m X 0.25 mm with 0.25 μm film thickness). Helium was used as the carrier gas for gas chromatography at a constant rate of 1 mL/minute. The injector was operated at 200°C and the temperature was maintained within 50-250°C at 10°C/min injection mode. Following MS conditions were utilized: ionization voltage of 70 eV, ion source temperature of 250°C, interface temperature of 250°C and mass range of 50-600 mass units (Sivaraj et al. 2019). NIST database was used for interpretation of compounds.
Phytochemical profile:
Secondary plant metabolites are distributed widely in different varieties of fruits and vegetables. In this study, qualitative analysis of phytochemicals report the presence of alkaloids, phenols, flavonoids, glycosides, saponins, tannins and quinones in both the extracts. Terpenoids were detected only in peel extract and cardiac glycosides were found only in pulp extract. TPC and TFC of the extracts were estimated using the regression equation obtained from the calibration curve of standards used for quantification of TPC and TFC. TPC and TFC of pulp extract were found to be 106.20 ± 1.13µg/mg GAE and 56.89 ± 3.66µg/mg QE respectively. TPC of peel extract was nearly three times greater (313.35 ± 4.03 µg/mg GAE) than the pulp extract. Similarly, TFC of peel extract was twice the amount (121.55 ± 3.66 µg/mg GAE) when compared to pulp extract.
Antioxidant activity:
Antioxidant activity of pulp and peel extracts of Docynia indica was determined using in vitro antioxidant assays (Table 1 and Table 2). Although, DPPH● radical scavenging potential was observed in both the extracts, the peel extract showed stronger activity as the IC50 value was 23.76 ± 3.38 µg/mL. Maximum inhibition was 91.65±0.06% at 60µg/mL. The IC50 value of pulp extract was 162.70 ±11.22µg/mL. A similar trend of results was noted for reducing power. Reducing power of the extracts was determined using Fe3+ reduction and phosphomolybdenum reduction methods. The reducing power of peel extract was high when compared to the pulp extract.
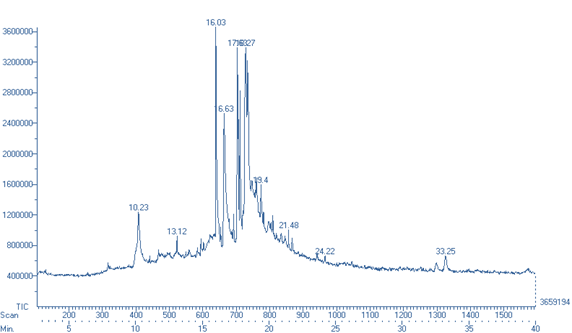
Figure 1: GC-MS chromatogram of pulp extract of D. indica
Table 4: GC-MS analysis of pulp extract of D. indica
|
Retention time |
Name |
Structure |
Molecular weight |
Molecular formula |
|
10.23 |
(4,4-dimethyl-5-methylene-4,5-dihydro-3H-pyrol-2-yl)-(4,4-dimethyl-5-methylene-pyrrolidin-2-ylidene)-acetonitrile |
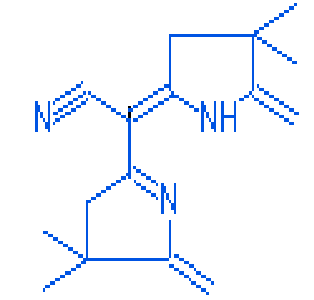 |
255 |
C2H3N |
|
13.12 |
Z,E-2-methyl-3,13-octadecadien-1-ol |
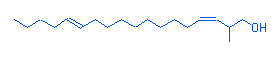 |
280 |
C19H36O |
|
16.03 |
Cyclopentaneundecanoic acid,methyl ester |
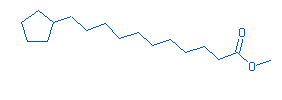 |
268 |
C17H32O2 |
|
16.63 |
9-oximino-2,7-diethoxyfluorene |
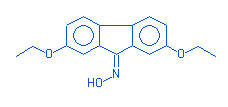 |
283 |
C17H17NO3 |
|
17.63 |
Oleic acid |
 |
282.00 |
C18H34O2 |
|
18.28 |
4H-1-benzopyran-4-one,3,5,7-trimethoxy-2-phenyl |
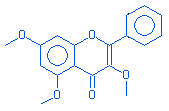 |
312.00 |
C18H18O5 |
|
19.4 |
Methoxyacetic acid, heptadecyl ester |
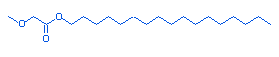 |
328 |
C16H32O3 |
|
21.48 |
Carbamic acid, N-methyl – N-[ 6-iodo-9-oxabicyclo(3.3.1) nonan-2-yl]-,ethyl ester |
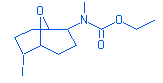 |
353.00 |
C12H20INO3 |
|
24.22 |
Pagicerine |
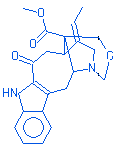 |
380.00 |
C22H24N2O4
|
|
33.25 |
Ursodeoxycholic acid |
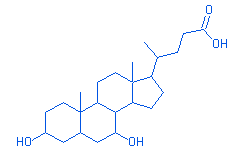 |
392.00 |
C24H40O4 |
Spectral analysis of ethanolic pulp extract of D. indica is depicted in figure 2 and given in table 5.
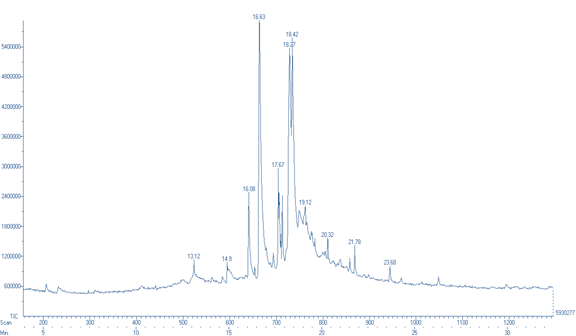
Figure 2: GC-MS chromatogram of peel extract of D. indica
Table 5: GC-MS analysis of peel extract of D. indica
|
Retention time |
Name |
Structure |
Molecular weight |
Molecular formula |
|
13.12 |
1H-pyrazole,4,5-dihydro-1-phenyl |
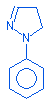 |
146 |
C21H18N2S |
|
14.9 |
Phenol,2,4-bis(1,1-dimethylethyl) |
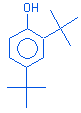 |
191 |
C17H30OSi |
|
16.08 |
Flavone |
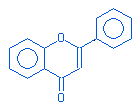 |
222 |
C15H10O2 |
|
16.63 |
n-Hexadecanoic acid |
 |
256 |
C16H32O2 |
|
17.67 |
Oleic acid |
 |
282 |
C18H32O2 |
|
18.27 |
6-octadecenoic acid |
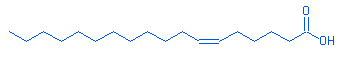 |
282 |
C18H34O2 |
|
18.42
|
Flavone,5,7-dihydroxy-8-methoxy |
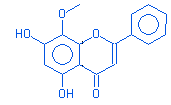 |
269 |
C16H12O5 |
|
19.12 |
Isopropyl stearate |
 |
326 |
C21H42O2 |
|
20.32 |
Coumarin,6-[7-hydroxycoumarin-8-yl]-7-methoxy |
 |
336 |
C9H6O2 |
|
21.78 |
Tricosane,2-4-dione |
 |
352 |
C23H44O2 |
|
23.68 |
Corynan-17-ol,18,19-didehydro-10-methoxy-,acetate [ester] |
|
368.00 |
C22H28N2O3 |
Providing scientific evidence on prophylactic therapeutic properties of wild edible fruits adds value to such wild plants that produce these fruit crops. This study evaluated the bioactive compounds and biological properties of pulp and peel extracts of D. indica. According to literature, the concentration of non-nutritive nutrients and antioxidant properties vary greatly between peel, pulp and flesh of whole fruit (Lata et al. 2009; Manzoor et al. 2012). Results of the study indicate that antioxidant activity, total phenolic and total flavonoid content were higher in peels. TPC and TFC of peel extract were found to be thrice and twice the amount present in pulp extract. Peel extract showed stronger antioxidant activity at a smaller range of concentrations (20-120 µg/mL) and the IC50 value was 23.76 ± 3.38 µg/mL. Wolfe et al. (2003) compared the TPC, TFC and anythocyanin content of fruit pulp and peel extracts of four apple varieties. The results showed that peel extracts had a higher concentration of all the above mentioned phytonutrients. Concentration of flavonoid in peels was six times greater than in flesh. Likewise, a systematic investigation of antioxidant potential of three apples produced in the Western Himalayas by Dhyani et al. (2018) showed that the antioxidant activity, composition and concentration of phytochemicals were higher in peel extracts followed by whole fruit and pulp. Khanizadeh et al. (2008) reported that flavonol glycoside, a phenolic compound with antioxidant activity was found only in the outer pericarp of apples. Eberhardt et al. (2000) documented the antioxidant activity of Malus domestica peel extracts to be 83 μmol ascorbic acid equivalents. Since the amount of Vitamin C present in 100g of apple was around 5.7 mg, the authors of the study concluded that apart from Vitamin C, antioxidant activity of different parts of Malus domestica mainly depends upon the presence of other bioactive constituents.
Due to over exploitation of different parts of medicinal plants, the bioavailability of non- nutritive components with excellent restorative properties is limited. Phytochemicals present in agro waste products such as vegetable and fruit peels with high beneficial value are either discarded or not utilized efficiently (John et al. 2018; Begam et al. 2020). Fruit peels are rich in antioxidant compounds, organic acids and enzymes (John et al. 2017; Pathak et al. 2017). Currently, the role of fruit peels in food, nutraceutical and pharmaceutical industries has started gaining importance. To ensure appropriate use of these novel waste substrates and to enhance the bioavailability of these functional constituents, use of cost effective eco-friendly strategies is recommended for extraction purpose. Irrespective of apple cultivars, valorisation of apple by-products such as peels, seeds and pomace obtained during production of apple based processed products is necessary. The study highlights that peel of wild fruits can serve as valuable resources of active compounds.
Indigenous and underutilized fruits that belong to the genera Artocarpus, Docynia, Musa, Morus and Myrica that grow in North Eastern regions of India are consumed by the local inhabitants. These wild underutilized fruits are rich in phytonutrients that impart numerous health benefits (Barua et al. 2019). In this study, TPC and TFC of pulp extract of D. indica were106.20 ± 1.13µg/mg GAE and 56.89 ± 3.66µg/mg QE. Sharma et al. (2015) evaluated the phytochemical profile and antioxidant activity of five wild fruits cultivated in Manipur viz Averrhoa carambola, Docynia indica, Garcinia pedunculata, Garcinia xanthochymus and Rhus semialata. Antioxidant activity decreased from R. Semialata < D. Indica < G. xanthochymus < A. Carambola < G. pedunculata. TPC and TFC of D. Indica was 49.26 ± 4.21 mg/g GAE and 0.51 ± 0.07 mg/g QE respectively. In the present study, pulp extract showed higher antibacterial effect than the peel extract. Antibacterial activity of pulp extract was ranked in the following order S. aureus < B. substilis < E.coli < M. luteus < S. flexneri < K. Pneumoniae. Findings of the study are in agreement to the results of previous studies. Shende et al. (2016) in their study reported that different extracts of D. Indica had inhibitory effect against B. cereus, S. aureus, E.coli, P. aeruginosa and Y. enterocolitica. Punjabi et al. (2014) compared the antibacterial effect of commercially available gooseberry and Docynia indica juice. Results signified that both the juices showed strong antibacterial activity.
Major sub groups of polyphenols include coumarins, flavonoids, phenolic acids, stilbenes and tannins. Flavonoids are classified into five categories such as flavonols, flavones, flavanols, flavanones, isoflavonoids and anthocyanindins (Zhang et al. 2015). Polyphenols such as catechins, epicatechins, chlorogenic acid, cyaniding-3-O-galactoside, hydroxycinnamates, and phloridzin identified in different varieties of flesh and peels of apples are associated with various health benefits (Vrhovsek et al. 2004; Cuthbertson et al. 2012). Quercetin conjugates are exclusively present in apple peels [39]. Therapeutic properties of polyphenols found in apples include remarkable antioxidant activity by enhancing the action of glutathione S – transferase, decreasing the production of H2O2, preventing oxidative induced lipid and DNA damage, possess antiproliferative properties and delays intestinal glucose absorption. Decreasing post prandial blood glucose prevents development of hyperglycaemia and metabolic syndrome (Miene et al. 2009; Petermann et al.2009; Schulze et al. 2014). Components such as coumarin, phenols, pyrazole, flavone and flavone derivates were indentified in peels. Since the concentration of phytonutrients is high in the pericarp, the study emphasizes the importance of utilizing the whole fruit with the peel to derive maximum benefits from it.
In order to promote maximum utilization of fruit crops, the study aimed at determining the antioxidant and antimicrobial activity of pulp and peel extracts of Docynia indica. The results indicate that both the extracts were rich in bioactive compounds and contributed to antioxidant and antimicrobial activity. However, the peel extract possessed stronger oxidizing and reducing power due to higher phenol and flavonoid content. In order to support the role of underutilized wild edible fruits as potent source of functional foods, more in vitro and human interventional studies focussing on nutritional and medicinal properties of wild fruit varieties should be carried out. The biological properties of fruit peel extract signify the potential role of fruit peels in different industries.
Conflict of interest:
There is no conflict of interest
The first author (Ms. Sangeetha ST) thank Research Seed Grant, Centre for Research and Development of Women’s Christian College (Autonomous) Chennai, for partly funding the research work. The authors also thank the Director, Armats Biotek Training and Research Institute, Guindy, Chennai, Tamil Nadu, India for providing the necessary facilities.
Abbreviation
DPPH˙: 2, 2-diphenyl-1-picrylhydrazyl
FeCl3: Ferric chloride
GAE: Gallic acid equivalent
GC-MS: Gas chromatography mass spectrometry
K3 [Fe(CN)6]: Potassium ferricyanide
NaCO3: Sodium carbonate
Na2NO3: Sodium nitrate
NaOH: Sodium hydroxide
QE: Quercetin equivalent
TFC: Total flavonoid content
TPC: Total phenol content
ZOI: Zone of inhibition.
REFERENCES
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.