


Characterization of Indian Incense Stick Powders for their Physical, Chemical and Mineralogical Properties
Virendra Kumar Yadav1*, Bijendra Singh2, Nisha Choudhary3
1 School of Life and Basic Sciences, Jaipur National University, Jaipur, India
2 School of Chemical Sciences, Central University of Gujarat, Gandhinagar, India
3 School of Nanosciences, Central University of Gujarat, Gandhinagar, India.
Abstract
The incense sticks ash composition totally relies on the chemical composition of incense sticks and other incense products. In order to obtain the detailed properties of incense sticks ash, it is important to have detailed chemical, elemental and mineralogical properties of incense stick powders. Though the major chemical composition of most of incense stick powders is common, different brands slightly modify their composition, fragrance etc. Generally, incense stick powders comprises wood chips, fragrance material, coal powder and adhesive material. The chemical composition of incense powder has a role in the final texture, fragrance and properties of incense sticks. The characterization of raw incense stick powders by Fourier Transform Infrared Spectroscopy (FTIR), X-ray diffraction spectroscopy (XRD) and Field emission scanning electron microscopy (FESEM-EDS) revealed that the particles have numerous organic compounds as detected by FTIR. The XRD revealed the amorphous nature of the particles due to the presence of a large number or organic carbon. While FESEM revealed the micron-sized, amorphous and irregular-shaped particles. The information of incense powder will help in the deep insight of incense sticks ash.
Keywords: Incense stick powders; ambergris; operculum; hydrocarbons; volatile matters
Incense sticks are commonly used in the Indian subcontinent, Asian countries, and the USA (Friborg et al., 2008). The major composition of incense powder is almost constant for all types of incense irrespective of different industries and brands. Typically, an incense stick powder comprises fragrance material, wood chips, coal powder and adhesives (Tyagi & Kalauny, 2007). The role of fragrance material is to provide aroma to the incense products, which could be jasmine, rose, chameli, lavender, mogra, etc (Al-Seeni, 2017; Abbaszadeh et al., 2017). These fragrance material are generally derived from the plant extract or phytochemicals. Most of these plant materials are volatile organic matters (polycyclic hydrocarbons and polyaromatic hydrocarbons). The fragrant materials provide the aroma and produce the fragrant smoke when incense is burned. Many types of fragrant woods, resins, herbs, and essential oils are used as incense, individually or in combination. Many of the same fragrant materials are the same as those commonly used in perfume formulations.
The plant-derived materials can be used as a source of incense (Staub et al., 2011; Wei See et al., 2016) in various incense products like incense stick powders, dhoop, dhoop cones etc. Different parts of the plant, which are used are leaves, woods, barks, seeds, fruits, roots, and flower. These materials are broadly classified over here.
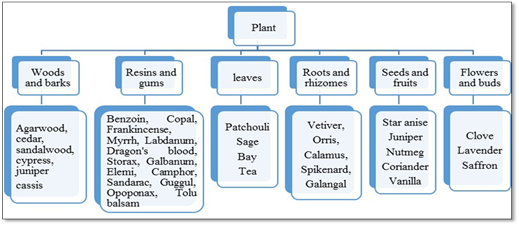
Fig. 1: Plant-derived materials used for the preparation of incense stick powders
Besides plant, there are numerous fragrance materials, which are derived from the animals. But their use may raise some ethical and religious issues as mainly the incense sticks are concerned with divine purposes. In comparison to plant only a few materials are used originated from animals for fragrance in incense, which are shown below in figure 4. The most widely-used, animal -originated incense fragrance materials are operculum (Nongmaithem et al., 2017), ambergris (Clarke, 2006; Brito et al., 2015), and musk (Elsayed et al., 2016). The operculum is derived from soft-bodied animals like snails, molluscs and mosses (Páll-Gergely et al., 2016; Klbrahim et al., 2018). The operculum is made up of a protein called “conchiolin”,(Prasuna et al., 2004; Curry et al., 1992), which is almost similar to the keratin. Keratin is the major component of nails and horns in animals and human beings. Musk is another animal-derived fragrance material, which is obtained from the male musk deer (Li et al., 2016; Meng et al., 2006). Musk is a heavy base note aromatic material, which is generally compared with the woodsy and earthy smells (Zarzo and Stanton, 2009; Benmiloud et al., 2019).
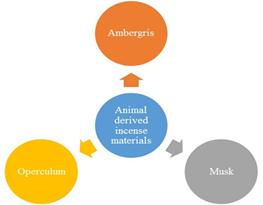
Fig.2. Animal-derived incense fragrance materials
It has got its name also because its odour is similar to that of produced by male musk deer. Another animal-derived fragrance material which is widely used in incense stick powders is ambergris. Ambergris is a waxy material that originates as a secretion in the intestine of the sperm whale, found floating in tropical seas (Srinivasan, 2015). Besides incense, it is also used in making perfumes and other aroma-based materials due to its strong odour.
Further, the wood chips and coal powder are mainly used as the base material, which ensures the complete burning of incense sticks (Patwardhan, 2005). Finally, the adhesive materials are used for providing adhesive properties to the incense powder, so that they can hold the coal powder, fragrance, and bamboo sticks together.
The composition of incense powder may further vary based on the fragrance materials used and some trade secrets used by the big players in the market in this field. Indian incense-based industries add diethyl phthalate (DEP) (Lin et al., 2008) to the powder paste in order to ensure the reduction of smoke released from the incense sticks burning. Similarly, ITC an Indian-based incense industry claimed to produce smokeless incense sticks. Further, in several countries, insect repellent substances are added to the incense powder in order to ensure the use of incense sticks as both fragrance material and insect repellant. Likewise, there are numerous modifications of incense powder around different parts of the globe, whose discussion will be out of context over here.
Incense stick powders generally have volatile matters which are oils, perfumes and other organic carbon sources (Jetter et al., 2012; Balasubramanian, 2015). The burning of these volatile matters produces 1/3 residue while rest are volatilized due to LOI (Loss on ignition). Different countries and industries have different compositions, but all of them have charcoal powder, fragrant materials, and adhesive gums. The detailed analysis of incense mix could help in minimizing the health issues related to burning to the smoke of incense sticks. Here, the incense stick powders were thoroughly dried and analyzed by the Fourier Transform Infrared Spectroscopy (FTIR), Particle size analyzer (PSA), X-ray diffraction spectroscopy (XRD) and Field Emission Scanning Electron Microscopy (FESEM-EDS).
Incense sticks of different colors, fragrance and brands, glass beaker, Grinder, Sieve sets, mortar-pestle
Incense sticks of different brands and color and fragrance were procured from the local market. The powder of incense sticks was separated from the incense sticks. The powder was collected while the bamboo sticks were discarded. The incense stick powders, which was collected, firstly, grounded in a grinder and later on, it was through a sieve. The powders were analyzed for their color, size and other properties.
The chemical properties of incense stick powders were evaluated by electrical conductivity (Analab), pH meter (Analab) for determining the pH of the aqueous paste. The FTIR measurements of incense stick powders were done by preparing KBr pellet technique and analysis was done by a Perkin-Elmer, spectrum 6500 instrument at a resolution of 2 cm-1. It was used for the detection of various functional groups and ultimately organic compounds present in the incense stick powders. The XRD analysis was done for the phase identification of various amorphous and crystalline phases present in the incense stick powders. The XRD patterns were recorded using a Philips X’PERT PRO instrument equipped X’celerometer in the 2θ range of 20-70 with a step size of 0.02 and a time of 5 seconds per step at 40 kV and a current of 30 mA. The morphological analysis of incense stick powders was done by Carl Zeiss, (NOVA, NANOSEM) FESEM, at variable magnification. The analysis was carried out by spreading powder on double-sided carbon tape and gold sputtering was done for 10 minutes.
Loss on ignition: About 10 grams of raw incense powder was taken and calcinated at 400 °C for two hours. The weight of the residue or ash was measured. About three grams of ash was obtained. One can say that the 70% fractions of incense powder are made up of volatile matters like oil, perfumes and other organic carbon products. The loss in weight was the loss on ignition of incense stick powders.
Their color generally depends on the additives and color used while preparation of incense powder. Based on the above factors it could be of blue, black, red, pink, etc. but the most common color is black. But, generally, the color of incense powder is black due to the use of coal powder, while in some incense stick powders wood chips powder is used, whose color is greyish.

Fig.3. A typical black color incense stick powders
The incense stick powders are fine particles, which have a homogenous texture. Besides, their texture depends on the composition and material used while preparation. The incense sticks, which wood chips and coal have been used in them (Lin et al., 2008) will have a rough texture as the coal powder and wood chip particles are coarser in size. Moreover, the coarser size helps in the smoother burning of incense sticks. Nowadays, some of the incense stick powders have cow dung with rice paddy or wheat husk, which are cheaper materials for incense-based industries (Mehra, 2011).
Various chemical matters affect to the chemical properties of incense in which alkali metal, metal oxides and aldehydes like acetaldehyde, acrolein, formaldehyde, furfural and aromatic hydrocarbons such as benzene, styrene, toluene, and xylene are involved (Derudi et al., 2012). These chemicals influence chemical properties like pH, electrical conductivity, etc. Here, we are going to discuss pH and electrical conductance on the basis of participating metals and hydrocarbons of incense.
The pH of the raw incense powder in distilled water is about 7.81. The pH is slightly basic; this could be due to the high amount of alkali metals like calcium and magnesium in the incense. The major alkali metals like Ca, Mg, k, and Na are mostly responsible for the basic nature of incense. Moreover, the electron-rich functional group may also affect the basic nature of the incense or even acidic nature in the case of electron-deficient chemical compounds.
The electrical conductivity of the raw incense powder solution is 0.0054 Siemens. The electrical conductivity depends on releasing capacity of the electrons and the alkali metals like Na, Ca, Mg, and K play a crucial role to direct the electrical conductance (Mähler and Persson, 2011) in incense. The elements like C, Ca, Mg, K, Si, etc. are generally out-sourced from the coal powder; C is from the oil or perfumes. The Indian incense paste has high Ca due to the addition of DEP to minimize the smoke. Additionally, the electron-rich species of the organic hydrocarbons present in incense also regulate the electrical conductivity.
The natural materials including incense, dhoop and cones have different organic chemical contestants along with involved metals. Such organic functional groups are responsible for the fragrance, conductance and also influences the pH of respective materials. Thus, it is very important to know about the significant role of such chemical compositions as organic functional structures. To investigate the functional groups of such natural chemical, FTIR technique is one of the useful tools, which helps to categorize the hydrocarbons and metal hydrocarbon species. Figure 4 shows the FTIR spectra of the raw incense to confirm the presence of hydroxyl, C-C, C=O and aromatic species. The OH group shows its characteristics in the FTIR spectra band around 3400 cm-1 due to the moisture or water molecule in the incense powder (Peng, wu, and Yang, 2003). The transmittance band at 1600 cm-1 is attributed to the C=O group due to the presence of various organic molecules present in the incense powder (León et al., 2017). The band from 1400-800 cm-1 is attributed to the aromatic compounds present in the incense powder.
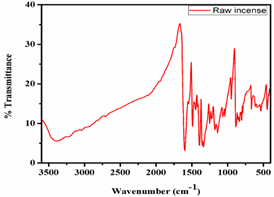
Figure 4. FTIR spectra of incense stick powders
The vibrational bands around 1373 and 2327 cm-1 are assigning to the presence of the carbon, hydrogen, and oxygen functional groups of other hydrocarbons (Chen et al., 2015). The transmittance peaks around 1372 and 1541 cm-1 indicate the stretching of C-H mode, H-O-H bending vibrations of water (Thompson et al., 2014) and C=O symmetric stretched vibrations due to carbon dioxide and carbonate anions.
X-ray diffraction (XRD) technique is highly applicable for determining the crystal structures, defects and elemental compositions of the materials. Regarding this, we went through the XRD analysis of the raw incense at room temperature at the range of 10-60 2θ degrees. Here, the lower-degree angle identifies that organic materials contents along with some alkali metals. It is clearly seen that the prominent peak around 13.4 2θ indicates the presence of carbon (Maruthapandi and Gedanken, 2019), the peak at 23.3 2θ is due to the silica (Kumar et al., 2016) and another high-intense peak at 29.61 2θ confirms the calcite composition along with the minor peak at 39.68 2θ degree (Peh et al., 2017). Further, the peak around 36.22 2θ is observed featured by the magnetite (Ouyang et al., 2015) contents with the 27 2θ peak position due to the quartz (Marinoni and Broekmans, 2013).
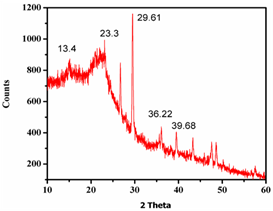
Fig.5: XRD spectra of incense stick powders
So, it can be concluded that the carbon source could be materialized through the organic contents in incense while the calcite, silica and iron oxide occurred due to the coal powder, which was significantly recognized by the XRD profile due to the presence of these higher concentration minerals in incense. From the XRD pattern, it is found that the diffraction peaks are having amorphous nature with the low intense peaks because of hydrocarbons and the light-weight metals in incense.
FESEM-EDS is one of the highly recommended tools for the structural and morphological studies of the materials. The size, shape and elemental compositions of the scanned materials could be precisely investigated using FESEM-EDS. We analyzed our materials using this technique at low KeV (5 KeV) energy so that our materials not to be damaged under applied energy during analysis. In the FESEM images, it is found that the particles are highly aggregated together to form a lump. Moreover, the incense stick powders are irregular in shape and size in fig 6a and b. Most of the light-colored bright area are carbon-rich particles (fig 6b) while the darker particles are electron-rich Fe, Al, Si-rich area. The size of the raw incense stick powders is generally varying in the micron range i.e. 1-10 microns.
Fig. 6. FESEM micrographs of incense stick powders at a) 5 microns b) 1 micron
In the present study, we reported an inclusive explanation about the incense materials including its physical and chemical properties, morphology, chemical compositions and significance. The physical properties are focused in terms of its pH and electrical conductance, which mostly occurs due to the presence of alkali metals into the incense. The FTIR analysis confirmed the possible vibration modes of involved chemical functional groups in the form of OH, C=O and other hydrocarbons. Additionally, the XRD results reflect a crystalline nature of the raw incense by indicating carbon, silica, calcite, and iron oxide at a higher amount. The FESEM revealed the irregular, amorphous, carbon-rich and micron-sized particles in the incense stick powders.
REFERENCES
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.